Abstract
While CAR T cell therapy can be curative for some, clinical data suggest that T cell dysfunction after infusion is a central cause of the therapeutic failure that occurs for most patients. Recent studies have demonstrated that chronic CAR activation, either from antigen-independent signaling (Long AH, Nature Medicine 2015; Lynn R, Nature 2019) or persistent exposure to antigen (Singh N, Cancer Discovery 2020; Good CR, Cell 2021) can lead to T cell dysfunction. Whether all CAR-driven T cell dysfunction results from the same cellular mechanisms remains unknown. We sought to directly interrogate the contribution of costimulatory domains, the primary structures differentiating approved CAR T cell products, to CAR-driven dysfunction.
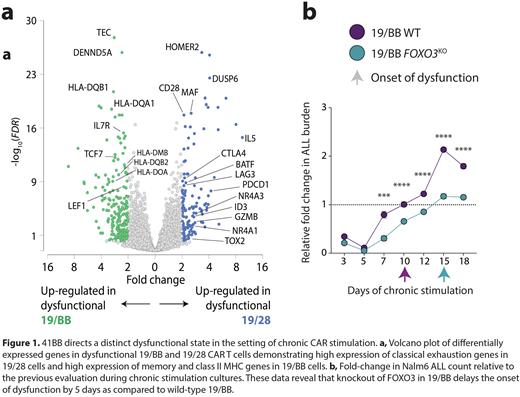
To undertake this, we established an in vitro model that recreates prolonged anti-CD19 CAR stimulation and performed integrated functional and genomic profiling of CD28 or 41BB-based (19/28 or 19/BB) CAR T cells over time. Both cell types were initially able to control high quantities of tumor but became dysfunctional after ~10-12 days of chronic stimulation. This dysfunction manifested as an inability to kill target cells, secrete cytokines, expand and persist, mirroring defects in function seen in patients who do not have responses. RNA sequencing revealed that 19/28 and 19/BB had very similar transcriptional profiles at rest and during activation but diverge as they lost function. Dysfunctional 19/28 cells bore the hallmarks of classical T cell exhaustion, expressing high levels of PDCD1, CTLA4, TOX and NR4A. In contrast, dysfunctional 19/BB cells preferentially expressed class II MHC genes as well as genes associated with T cell memory, such as LEF1, TCF7 and IL7R (Figure 1a). Evaluation of chromatin accessibility using ATAC sequencing revealed a similar trend, with opening of loci associated with exhaustion only in 19/28 cells.
To further clarify the transcriptional identity of dysfunctional 19/BB cells we performed single cell RNA sequencing of both cell types at rest, during activation and after onset of dysfunction. These data revealed that dysfunctional 19/BB cells were a heterogenous population, wherein some cells expressed class II MHC genes but the majority (70%) belonged to a unique cluster defined by expression of GNLY, CCL5, KLRK1, GZMA and ID2. Single cell RNA sequencing of CAR+ T cells from a patient with diffuse large B cell lymphoma who received Kymriah, a 19/BB-based CAR T cell product, demonstrated consistent transcriptional evolution. This patient had a partial response one month after treatment with disease progression at three months. We evaluated circulating CAR+ T cells at days 14 and 100 after treatment and observed that day 100 cells were also defined by high expression of GNLY, CCL5, KLRK1 and GZMA.
We next sought to identify the transcriptional regulators that led to 19/BB dysfunction. Using our ATACseq data we performed motif analysis to identify which transcription factor binding sites were opening as each cell type progressed from rest to dysfunction. As has been previously shown (Lynn R, Nature 2019), 19/28 cells demonstrated opening of sites for AP-1 factors Jun and Fos. 19/BB cells, instead, had increased accessibility at binding sites for forkhead box-O (FOXO) proteins. Consistent with these data, our single cell RNAseq demonstrated increased expression of FOXO3 target genes in dysfunctional 19/BB cells, confirming not only increased accessibility but also increased FOXO3 activity in these cells. To determine if FOXO3 was responsible for failure of 19/BB cells we disrupted the FOXO3 locus in T cells expressing either 19/28 or 19/BB and subjected these cells to our chronic stimulation cultures. Intriguingly, disruption of FOXO3 consistently delayed the onset of dysfunction in 19/BB but not 19/28 cells (Figure 1b).
Collectively, these data suggest that costimulatory domains play a central role in CAR-driven T cell dysfunction that leads to therapeutic failure. While CD28-based CARs promote classical exhaustion programs, 41BB-based CARs drive a dysfunctional trajectory driven by re-activation of FOXO proteins. Development of strategies that can bypass both forms of failure will be critical to improving the efficacy of this platform.
Disclosures
Selli:Wugen: Current Employment. Fehniger:BMS: Other: Provision of drug for investigator initiated trial; ImmunityBio: Research Funding; HCW Biologics: Research Funding; Orca Bio: Current holder of stock options in a privately-held company; Indapta: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board; Affimed: Other: Scientific Advisory Board, Research Funding; Wugen: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties. Singh:Novartis: Patents & Royalties; Rivervest Ventures: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal